Separation by fractional distillation
- Fractional distillation is the process of separation two or more miscible liquids by distillation, the distillate being collected in fractions boiling at different temperatures.
- The separation of two liquids by fractional distillation depends on the difference in their boiling point, where the boiling point difference must be less than.
- Air is a homogeneous mixture of gases like nitrogen, oxygen, carbon dioxide, argon, etc. which are present in different proportions.
- Special separation techniques are required for homogeneous mixtures.
- Fractional distillation is the technique that is used for obtaining different components from the air.
- In most simple terms, a fractionating column can be regarded as an arrangement for providing different temperature zones inside it (during distillation), the highest temperature being at the bottom of the column and the lowest temperature near its top.
Applications of Fractional Distillation
- Fractional distillation is used to separate mixture of miscible liquids (like alcohol-water mixture and acetone-water mixture) in the laboratory.
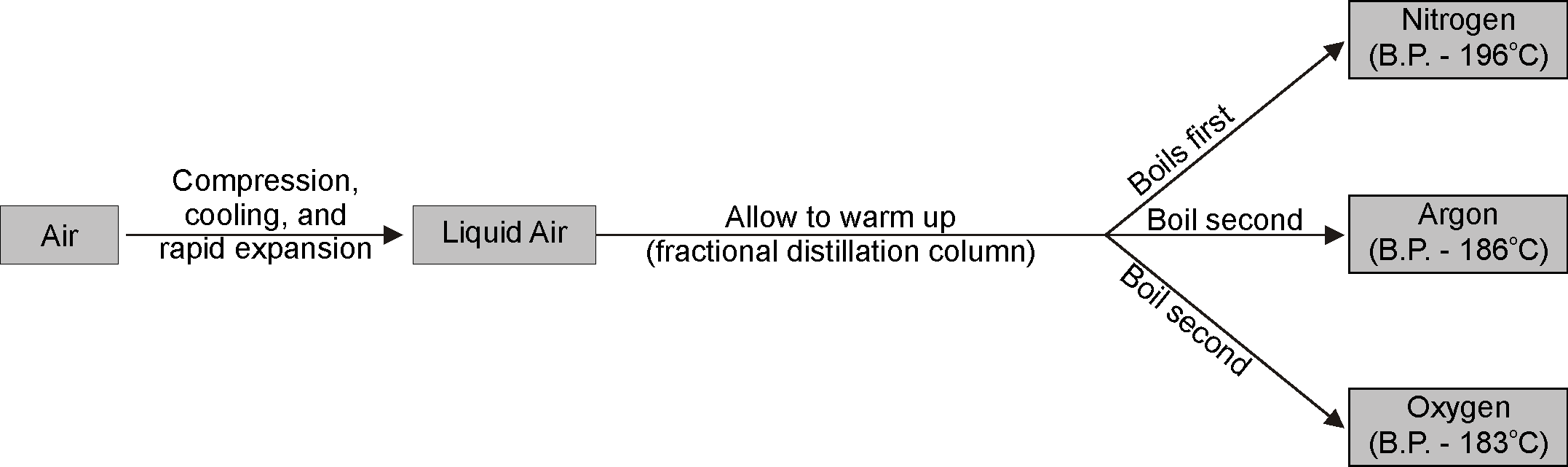
- Fractional distillation (of liquid air) is used to separate gases of the air.
- The difference in boiling points of components is used to separate the liquid mixture into fractions through distillation.
- Consider the following illustration of separation of nitrogen from the air.
- In order to obtain nitrogen gas from air, rest of the constituents of air need to be removed. So, air is filtered to remove the dust particles and then liquefied.
Step 1: Conversion of air into liquid air
The air which is in gaseous form is converted into liquid air under high pressure. Due to the high pressure, the air is compressed and then cooled by reducing the temperature resulting in liquid air.
Step 2: Fractional Distillation
The liquid air is then passed through the fractional distillation column where the liquid air is allowed to warm-up. The bottom of the fractionating column is warmer than the top. Gases begin to separate at different temperatures according to its boiling point.
Nitrogen has a boiling point of
while oxygen has
. So, the nitrogen gas will start to escape through the outlet and it is collected. Liquid oxygen will be collected in the fractionating column.