Characteristics of the Particles of Matter:
- Substances are made up of particles that exhibit some characteristics which can influence the state and properties (physical and chemical) of a substance.
- The following three characteristics are shown by particles of matter:
- PARTICLES HAVE SPACE BETWEEN THEM
- Every particle in a matter has some small voids in between.
- This characteristic determines the solubility of a substance in other substances.
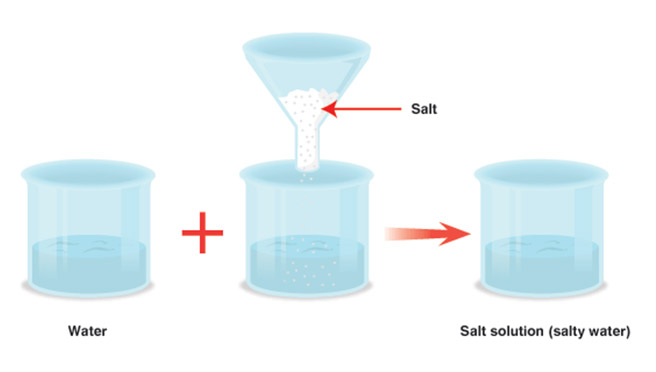
- If a teaspoon of sugar is put a glass of water and mixed properly the water level remains the same..
- This is because the particles of sugar get into the inter-particle spaces between the water molecules.
- This proves the presence of voids between particles of a substance.
- But on adding more salt or sugar, it will dissolve until all the space between water particles gets filled.
PARTICLES ARE CONTINUOUSLY MOVING
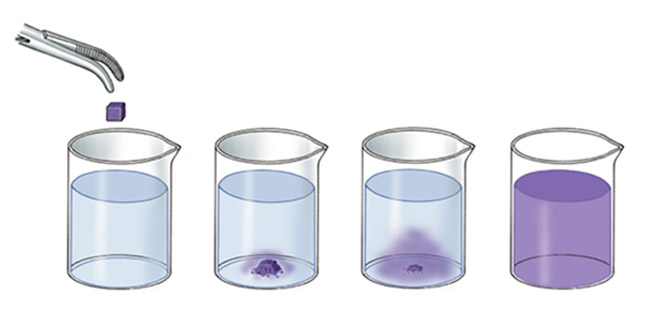
- Particles of the matter exhibit continuous random movements due to their kinetic energy.
- For example, the spreading of ink in a beaker of glass, the smell coming from incense stick (agarbattis), etc. shows the particles of a substance move.
- Diffusion is the phenomenon in which the particles of two different types of matters intermix on their own.
PARTICLES ATTRACT EACH OTHER
- There is an inter-particle force of attraction acting between the particles of every substance which needs to be overcome in order to break it.
- The strength of the force differs from one substance to another.
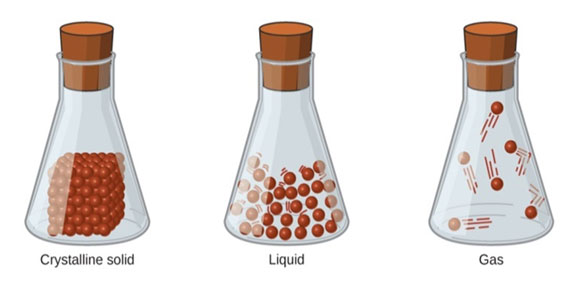
- The physical state of any matter is determined primarily by the inter-particle force of attraction and the kinetic energy of the particles.
- The force of attraction is maximum in the particles of solid matter and minimum in the particles of gaseous matter.