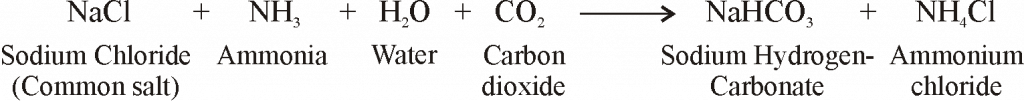SODIUM CARBONATE (WASHING SODA)
It is a whitish, odorless powder. Its chemical formula is Na2CO3.
PRODUCTION OF SODIUM CARBONATE BY SOLVAY’S PROCESS
Washing soda is produced from sodium chloride (or common salt) by Solvay’s process involves the following three steps:
- A cold and concentrated solution of sodium chloride (called brine) is reacted with ammonia and carbon dioxide to obtain sodium hydrogencarbonate. Carbon dioxide involved is produced through calcium carbonate and the calcium oxide left is used in recovering ammonia from ammonium chloride. :