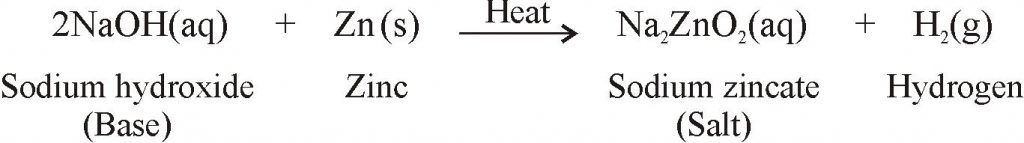
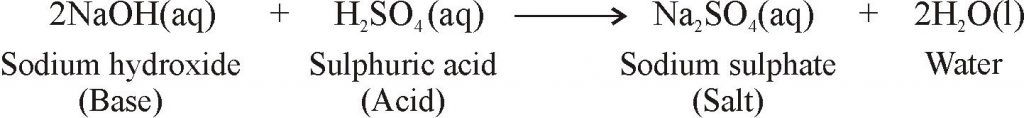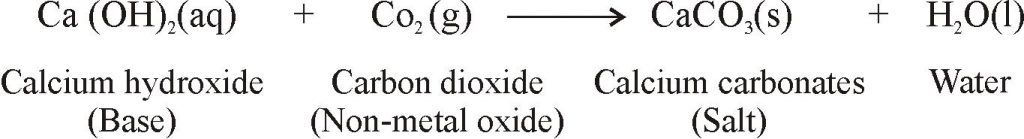REACTION OF BASES
REACTION OF BASES WITH ACIDS TO FORM SALT AND WATER
- Reaction of base with acid results in the formation of salt and water.
- For example, when sodium hydroxide reacts with the sulphuric acid, then sodium sulphate and water are formed: