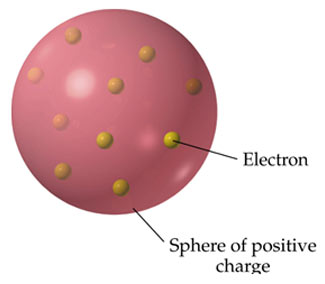
Postulates of Thomson’s Atomic Model:
Limitations of Thomson’s Atomic Model:
The following are the drawbacks of Thomson’s atomic model:
- The model of atom failed to explain how a positive charge holds the negatively charged electrons in an atom. So, it failed to explain the stability of an atom.
- This theory also failed to account for the position of the nucleus in an atom.
- Thomson’s model failed to explain the scattering of alpha particles.
- Even though Thomson’s model was not an accurate to account for the atomic structure, it proved to be the base for the development of other atomic structure models.