Consider the following examples.
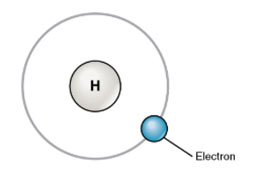
Electronic configuration of Hydrogen:
Atomic number = 1
K – shell / 1st Orbit: n = 1
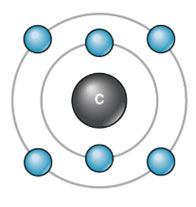
Electronic configuration of Carbon:
Atomic number = 6
K – shell / 1st Orbit = 2
L – shell / 2nd Orbit = 4
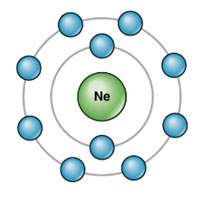
Electronic configuration of Neon:
Atomic number = 10
K – shell / 1st Orbit = 2
L – shell / 2nd Orbit = 8