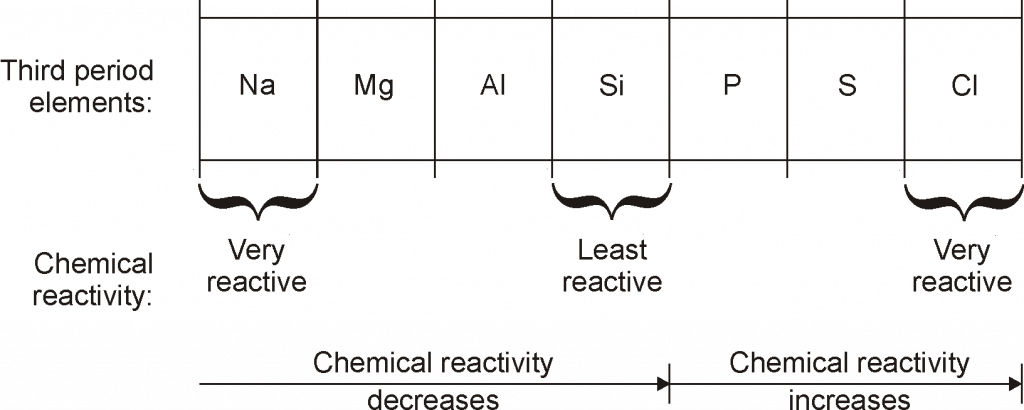
CHARACTERISTICS OF PERIODS
Valence Electrons (or Outermost Electrons)
- On moving from left to right in a period, the number of valence electrons in elements increases from 1 to 8.
Size of Atoms (or Atomic size)
- On moving from left to right in a period, the size of atoms(atomic size) decreases.
CHARACTERISTICS OF GROUPS:
Valence Electrons (or Outermost Electrons)
- All the elements of a group of the periodic table have the same number of valence electrons.
- For example, group I elements: Li, Na, K etc., all have a valency of . Elements of group 17: Cl, Br, I etc., all have valency of .
Size of Atoms (or Atomic size)
- While going down in a group of the periodic table, the size of atoms increases (or atomic size increases). This is due to the addition of new electronic shells.