GRAM ATOMIC MASS AND GRAM MOLECULAR MASS
Gram Atomic Mass:
The amount of a substance whose mass in gram is numerically equal to its atomic mass, is called gram atomic of that substance.
Atomic mass of oxygen, O = 16 u
Gram atomic mass of oxygen, O = 16 u grams
The gram atomic mass of a substance represents the mass of 1 mole of atoms
of that substance. The molar mass of an element is the mass of 1 mole of its atoms. The molar mass of an element has
atoms of the element in it.
Gram Molecular Mass:
The amount of a substance, whose mass in gram is numerically equal to its molecular mass, is called gram molecular mass of that substance.
Molecular mass of oxygen,
Gram molecular mass of oxygen,
grams
The gram molecular mass of a substance represent the mass of 1 mole of molecules
of that substance.
MOLE CONCEPT
particles (atoms, molecules or ions) of a substance is called a mole of that substance.
, which represents a mole, is known as Avogadro number (NA).
Mole of Atoms:
Mole of Molecules:
Applications of mole concept:
If mass is given and moles or number need to be calculated follow the below steps:
MOLE CONCEPT CALCULATION
Conversion of Moles to Mass and Vice Versa:
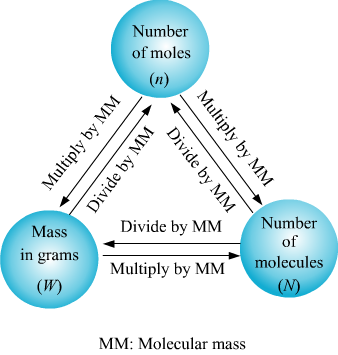
The key concept used in these kind of problems is that a mole of any substance contains gram formula mass or molecular mass of that substance.
For example, molecular mass of Hydrogen is 2 a.m.u. so mass of 1 mole of hydrogen which is also known as molar mass will be 2 gram. Similarly if we have 2 moles of hydrogen, it will weigh grams which is equal to 4 grams.
PROBLEMS BASED ON MOLES OF MOLECULES
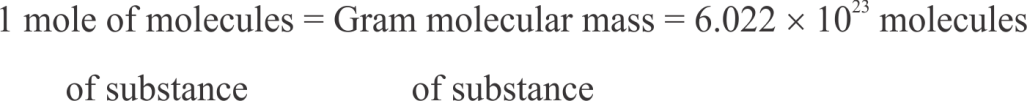
which is called Avogadro number or Avogadro constant.