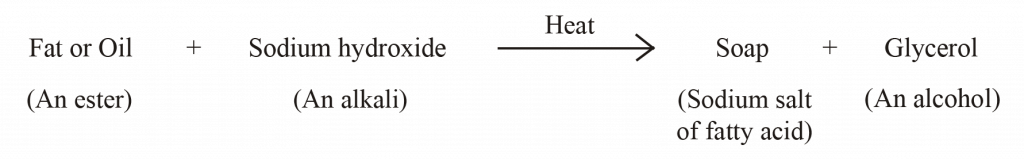


DIFFERENCES BETWEEN SOAPS AND DETERGENTS
| Soaps | Detergents |
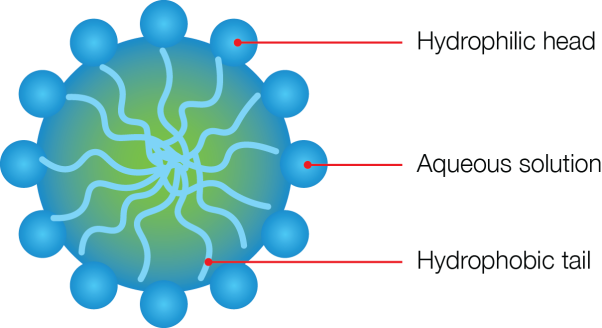
| Soaps are the sodium salts (or potassium salts) of the long chain of carboxylic acids (fatty acids). The ionic group in soaps is – COO-Na+ | Detergents are the sodium salts of long chain benzene sulphonic acids or long chain alkyl hydrogen sulphates. The ionic group in a detergent is –SO3-Na+ |
| Soaps are not suitable for washing purposes when the water is hard. | Detergents can be used for washing even when the water is hard. |
| Soaps are biodegradable. | Some of the detergents are not biodegradable. |
| Soaps have relatively weak cleansing action. | Detergents have a strong cleansing action. |