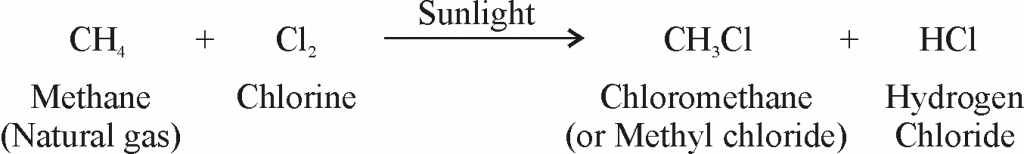
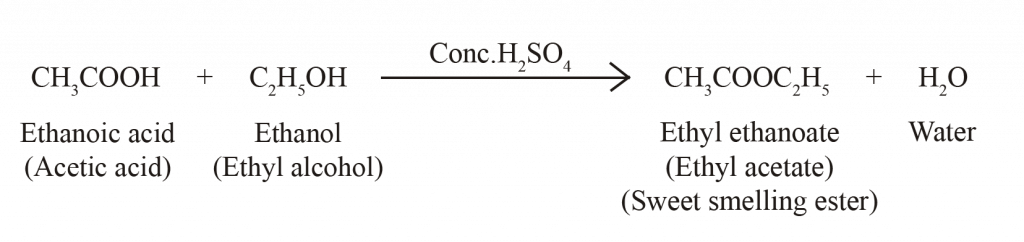
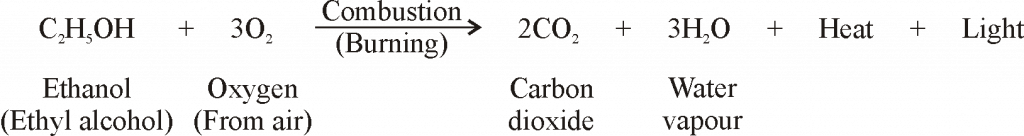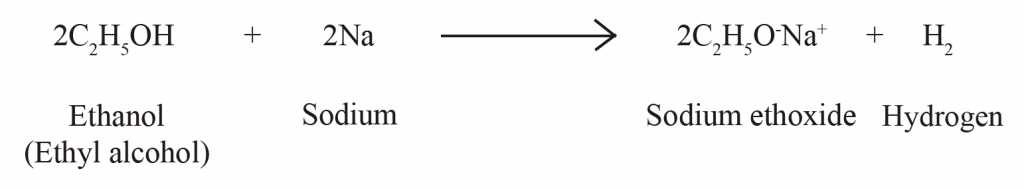SOME IMPORTANT CARBON COMPOUNDS
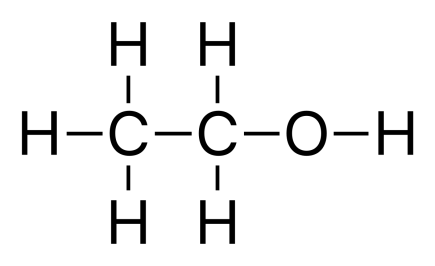
CHEMICAL PROPERTIES OF ETHANOL
REACTION WITH UNSATURATED HYDROCARBONS
- When ethanol is heated at 443 K in presence of excess conc. sulphuric acid alkene is obtained.
Ethyl alcohol Ethene water
- Water is removed by sulphuric acid which acts as a dehydrating agent.